Visualizing single molecules in whole cells with a new spin
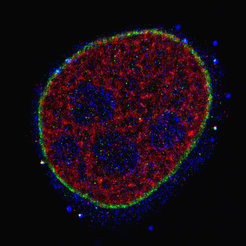
Three-color Exchange-PAINT image using a Spinning Disk Confocal Microscope super-resolves the microtubule network (green), TOM20 (red, mitochondrial outer membrane marker), and HSP60 (blue, heat shock protein located in the mitochondrial inner matrix) inside a HeLa cell.
Ralf Jungmann, Ph.D., head of the research group "Molecular Imaging and Bionanotechnology" at Max Planck Institute (MPI) of Biochemistry in Germany, Professor of Biochemistry at the Ludwig Maximilian University (LMU) and alumnus of the Wyss Institute and the Wyss Institute Core Faculty member Peng Yin, Ph.D. have been developing DNA-PAINT, a powerful molecular imaging technology that involves transient DNA-DNA interactions to accurately localize fluorescent dyes with super-resolution. However, although the researchers demonstrated DNA-PAINT’s potential by visualizing single biomolecules such as proteins in fixed cells at a fixed close distance, the technology could not yet investigate molecules deep inside of cells.
Now, Jungmann’s and Yin’s teams jointly report a solution to overcome this limitation. In their new study, they adapted DNA-PAINT technology to confocal microscopes, which are widely used by researchers in cell biology laboratories to image whole cells and thicker tissues at lower resolution. The MPI/Wyss Institute team demonstrates that the method can visualize a variety of different molecules, including combinations of different proteins, RNAs, and DNA throughout the entire depth of whole cells at super-resolution. Published in Nature Communications, the approach could open the door for detailed single-molecule localization studies in many areas of cell research.
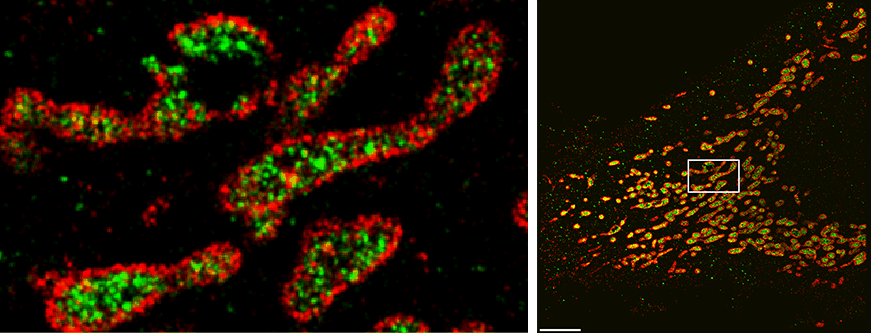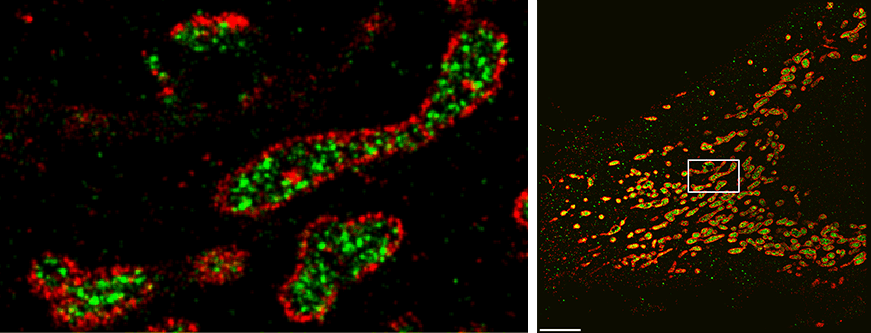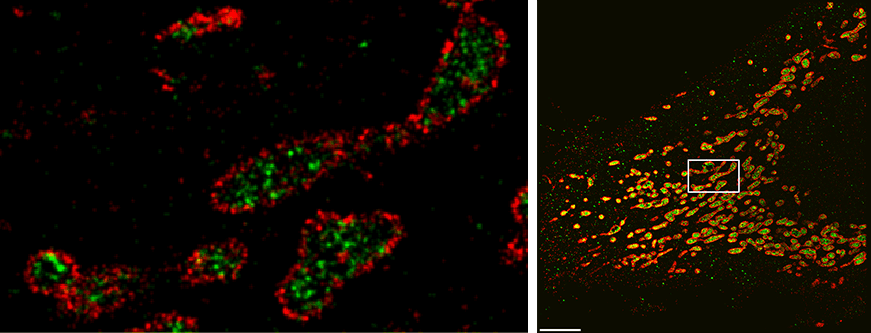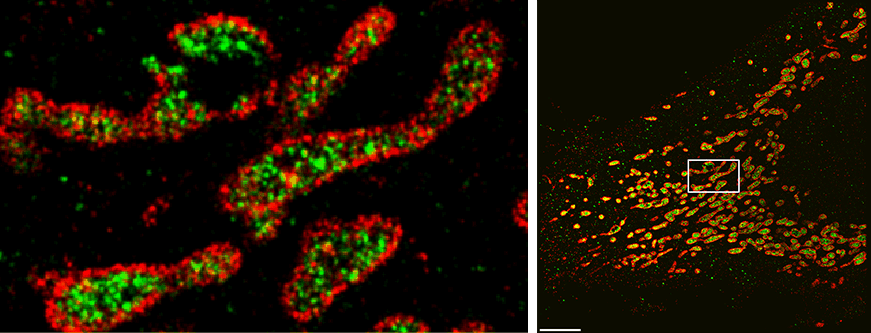
The DNA-PAINT approach attaches a DNA “anchor strand” to the molecule of interest. Then a dye-labeled DNA “imager strand” with a complementary sequence transiently attaches to the anchor and produces a fluorescent signal, which occurs as a defined blinking event at single molecular sites. Because this “blinking frequency” is precisely tunable, molecules that are only nanometers apart from each other can be distinguished — at the higher resolution end of super-resolution.
“Our new approach, SDC-PAINT, integrates the versatile super-resolution capabilities of DNA-PAINT with the optical sectioning features of confocal microscopes. We thus created the means to explore the entire depth of a cell, and to visualize the molecules within it at the nanometer scale,” said Jungmann. The team mapped out the presence of different combinations of proteins within whole cells, and then went beyond that. “By diversifying our labeling approaches, we also visualized different types of individual biomolecules in the chromosome-containing nucleus, including sequences in the DNA, proteins bound to DNA or the membrane that encloses the nucleus, as well as nuclear RNAs,” adds Yin, who is also co-leader of the Wyss Institute’s Molecular Robotics Initiative, and Professor of Systems Biology at Harvard Medical School.
In principle, confocal microscopes use so-called pinholes to eliminate unwanted out-of-focus fluorescence from image planes above and below the focal plane. By scanning through the sample, plane after plane, researchers can gather the desired fluorescence signals emitted from molecule-bound dyes over the entire depth. Specifically, the MPI/Wyss Institute team developed the technique for “Spinning Disk Confocal” (SDC) microscopes that detect fluorescence signals from an entire plane all at once by sensing them through a rotating disc with multiple pinholes. Moreover, “to achieve 3D super-resolution, we placed an additional lens in the detection path, which allows us to archive sub-diffraction-limited resolution in the third dimension” said first author Florian Schueder, a Graduate Student working with Jungmann who also worked with Yin’s Wyss Institute team as part of his master’s thesis.
“This addition can be easily customized by manufacturers of SDC microscopes; so we basically implement super-resolution microscopy without complex hardware changes to microscopes that are generally available to cell biologists from all venues of biomedical research. The approach thus has the potential to democratize super-resolution imaging of whole cells and tissues,” said Jungmann.
“With this important advance, super-resolution microscopy and DNA-PAINT could become more accessible to biomedical researchers, accelerating our insights into the function of individual molecules and the processes they control within cells,” said Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children’s Hospital, as well as Professor of Bioengineering at Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS).
Other authors on the study are past and present members of Yin’s group including Juanita Lara-Gutiérrez; Brian Beliveau, Ph.D.; Sinem Saka, Ph.D.; and Hiroshi Sasaki, Ph.D.; and Johannes Woehrstein, Maximilian Strauss, and Heinrich Grabmayr, Ph.D., who are working with Jungmann. The study was funded by grants from the Wyss Institute for Biologically Inspired Engineering at Harvard University, the German Research Foundation’s Emmy Noether Program, the European Research Council, LMU’s Center for Nanoscience, the Max Planck Society and Max Planck Foundation, the National Institutes of Health and the Office of Naval Research.
by Benjamin Boettner
Original publication:
F. Schueder, J. Lara-Gutiérrez, B.J. Beliveau, S.K. Saka, H.M. Sasaki, J.B. Woehrstein, M.T. Strauss, H. Grabmayr, P.Yin & R. Jungmann: “Multiplexed 3D super-resolution imaging of whole cells usingspinning disk confocal microscopy and DNA-PAINT”, Nature Communications, December 2017
DOI: http://dx.doi.org/10.1038/s41467-017-02028-8












