Two molecular machines caught in the act with the electron microscope
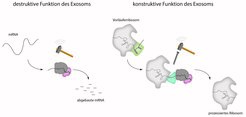
The exosome is a barrel-shaped molecular machinery through which the mRNA is threaded before it is completely degraded at the bottom by a nuclease. In addition to the degradation of RNA, the exosome also has a function in the production process of the ribosomes. The exosome deliberately degrades a part of the precursor ribosome.
Ribosomes, the protein factories of the cell, assemble protein building blocks into long chains based on a construction manual, the messenger RNA or mRNA. These chains are later folded into the protein. Faulty mRNA or RNA that is no longer needed must be degraded. This task is performed by the exosome. Previous studies have investigated the destructive function of the exosome, which – comparable to a destruction worker – completely degrades the RNA. In contrast, the now published study had a closer look at the precision work, i.e. the constructive function, of the exosome.
The exosome’s precision work
Ribosomes themselves are large protein complexes that must be produced in the nucleus. During this maturation process, the exosome binds to a precursor of the ribosome and selectively degrades a protruding part of the ribosome. A team of scientists led by Elena Conti, director of the department "Structural Cell Biology" at the Max Planck Institute of Biochemistry, and Ed Hurt from the University of Heidelberg have now been able to investigate this interaction of exosome and ribosome structurally. "This is comparable to the work of a stonemason who uses a hammer and chisel to remove stone in a targeted manner, thus creating a precise structure," explains Sebastian Falk, one of the first authors of the study alongside Jan Schuller.
Exosome and ribosome caught in the act
The researchers studied the two molecular machines by using the method of cryo-electron microscopy (cryo-EM), which was awarded the Nobel Prize in 2017. To this end, exosome and ribosome were shock-frozen together at very low temperatures. The scientists calculated the structure from the cryo-EM data and combined it with crystal structures from previous studies of the Conti department to generate a model.
Exosome expert Elena Conti explains: “One of my long-standing goals was to capture a snapshot of the exosome with a cellular substrate.” Now, Conti and her team have essentially caught the exosome and ribosome as they interact and function together. "This study is the culmination of many years of hard biochemical and structural work," adds Conti.
A new area of structural biology
Previous studies in the field of structural biology have always focused on individual molecular machines. “We now succeeded for the first time to visualize two molecular machines together. Thus, we no longer looked at them individually in isolation, but investigated their interaction. With this we take the physiological environment into account, marking this study as the beginning of a new area in the field of structural biology”, Conti explains the significance of this publication.
SSch/SiM
Original publication:
J. Schuller, S. Falk, L. Fromm, E. Hurt, E. Conti: Structure of the nuclear exosome captured on a maturing preribosome, Science, März 2018
DOI: 10.1126/science.aar5428












