Building muscle – one splice at a time
Max Planck Scientists discover important mechanism for building muscle
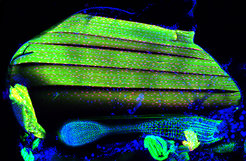
Every muscle is built from hundreds of contractile units, called sarcomeres. Different types of muscles use different protein variants to build these sarcomeres, resulting in muscles that run faster or slower or apply stronger or weaker forces. For instance, the flight muscles of fruit flies must contract 200 times per second to enable flight. Scientists in the team of Frank Schnorrer studied this model to address the question how a particular composition of sarcomeres is achieved.
“We were able to demonstrate that more than 700 proteins were present in different variants in the fast flight muscles as compared to the slow leg muscles,” explains Maria Spletter, first author of the study. “Thus, these protein variants form sarcomeres with different mechanical properties, which are adjusted to the needs of the respective muscle types.” One finding especially attracted the attention of the researchers: the protein variants were often generated from the same gene. Genes relate the construction plans for every protein. Broadly speaking, these construction plans are differently interpreted by a mechanism known as “alternative splicing”: Genes are made of small, modular units, called exons, which are transcribed by the cell, glued together and then translated into one protein. Alternative splicing links exons in various combinations and thus generates several protein variants from one single gene.
Stretched to rupture
However, how does the muscle know which variant of the protein is needed? In subsequent experiments, the scientists identified a protein called “Arrest” as an essential regulator of alternative splicing in flight muscles, gluing the correct exons together. “When Arrest function is deleted, flight muscles are primarily in the leg muscle splicing mode and form the wrong protein variants normally only present in leg muscle,” explains Spletter. The consequences are dramatic: these flies cannot fly anymore, and even worse, they destroy their flight muscles due to a condition termed hyper-contraction, during which their muscles are pulled to pieces by forces that are way too high.
These results may also be relevant for humans. “As alternative splicing is also important during heart and skeletal muscle development in humans and proteins similar to Arrest are present in mammalian muscles, this mechanism might also occur in humans,” Schnorrer speculates. Therefore, the discovery of this mechanism raises new questions, which will be addressed by the researchers in the future. “Studying Arrest biology in fruit flies can be an entry point to understand how alternative splicing of sarcomeric proteins contributes to healthy and diseased muscles in both flies and man.” [HS]
Original Publication
M. Spletter, C. Barz, A. Yeroslaviz, C. Schönbauer, I. Ferreira, M. Sarov, D. Gerlach, A. Stark, B. Habermann and F. Schnorrer: The RNA binding protein Arrest (Bruno) regulates alternative splicing to enable myofibril maturation in Drosophila flight muscle. EMBO Reports, December 22, 2014. DOI: 10.15252/embr.201439791






