Max Planck researchers describe new molecular shuttle service
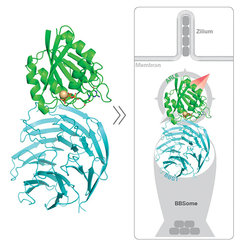
Once the BBSome has uptaken the protein supply for the cilium, ARL6 (green) binds to BBS1 (blue) thus directing the BBSome to its destination.
Cilia are tiny hair-like protein structures, which reside on the surface of almost all cells of both humans and animals. They carry out a number of important tasks, like the transmission of acoustic signals within the ear, optical signal in our eyes and the movement of sperm cells. This indicates how important these structures are. However, it also emphasizes how diseases which affect the cilia can severely impair the life of the respective patients. Bardet-Biedl Syndrome (BBS) is one such disease, in which affected individuals suffer from blindness and/or deafness, kidney failure and obesity. Until now, researchers were able to find out that BBS is caused by alterations of a certain group of nineteen proteins (BBS1-19). In healthy cells these proteins constitute a molecular shuttle that transports new building blocks towards the cilia – the so-called BBSome. In case the shuttle service does not work properly, the function of cilia is severely impaired. Researchers in the team of Esben Lorentzen at the MPI of Biochemistry recently identified the mechanism underlying the transport of the protein cargo in detail.
In the present study the scientists showed that the interaction of two particular proteins is necessary to deliver new building blocks to the cilia: Once the BBSome shuttle has uptaken its cargo, the molecule ARL6 is further needed to direct it to the cell surface where the cilia are located. ARL6 docks to a certain part of the BBSome, the protein BBS1, making sure that the shuttle is carried towards its correct destination. According to the researchers this mechanism is conserved from humans to green algae. This is usually good evidence, that a feature is essential for survival.
A molecular shuttle gone astray
Esben Lorentzen gives an example, how crucial this mechanism is for our health: “Interestingly, 30 percent of all BBS patients have a mutation at a certain position of BBS1, the consquences of which were not fully understood before. We were now able to show, that this mutation affects the binding between ARL6 and BBS1, inhibiting the interaction of those two molecules.” The scientists assume that this prevents the protein shuttle from being directed to its correct destination, subsequently leading to a lack of protein supply in the cilia and a loss of their function. In the future, the scientists hope to clarify whether other proteins are involved in the process and which potential roles they might play. [HS]



