Feeding RNAs to a molecular shredder - Max Planck scientists unravel the structure of a regulatory protein complex in RNA disposal
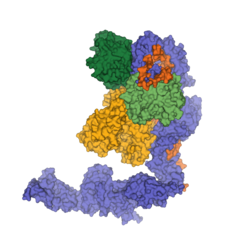
Any errors that occur during the synthesis of RNA molecules or unwanted accumulation of RNAs can be harmful for the cell. The elimination of defective RNAs or of RNAs that are no longer needed is therefore a key step in the metabolism of a cell. This task is carried out by a protein complex called Exosome, one of the research objects in the department “Structural Cell Biology” headed by Elena Conti. The molecular mechanism of how the Exosome is regulated, however, is not very clear yet.
The Max Planck scientists have now elucidated the atomic structure and the operating mechanism of a protein complex (Ski complex) which is involved in the activation of the cytoplasmic Exosome. The Ski complex contains several subunits and can be found in all eukaryotes – from yeast to humans. “We could show that the Ski complex and the Exosome interact directly and that they jointly form a channel for the RNA which is supposed to be degraded,” says Felix Halbach. Like DNA, RNA molecules are often folded. To be degraded by the Exosome, RNA molecules have to be unfolded first – this task is executed by the Ski complex. The unfolded RNA molecules can then be guided through the joint channel to the Exosome. “The Ski complex feeds RNA molecules to the Exosome,” explains the biochemist.
The results also reveal additional parallels between the Exosome and the Proteasome. The Proteasome is the protein complex responsible for the degradation of proteins in a cell. “It becomes clear that these two complexes are not only structurally and functionally similar,” says Elena Conti, “also their regulatory subunits work in a similar manner.” They unwind RNA molecules or, respectively, proteins and guide them to the active centers of the specific degradation machinery.
Original Publication
Halbach, F., Reichelt, P., Rode, M. and Conti, E.: The yeast Ski complex: Crystal structure and substrate channeling to the RNA exosome. Cell, August 15, 2013
DOI: 10.1016/j.cell.2013.07.017
Contact
Prof. Dr. Elena Conti
Structural Cell Biology
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
E-Mail: conti@biochem.mpg.de
www.biochem.mpg.de/conti
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Phone: +49 (0) 89 8578-2824
E-Mail: konschak@biochem.mpg.de
www.biochem.mpg.de












