Protein folding
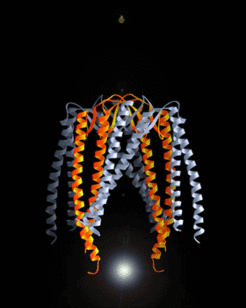
Prefoldin structure
Siegert et al., 2000, Cell 103, 621-632
In vitro studies over the last decades have outlined the basic mechanisms of two major chaperone systems that participate in protein folding in the cytosol, the Hsp70 proteins and the chaperonins. Relatively little is known, however, about the actual role of these components in protein folding in vivo. Major questions being addressed in the laboratory concern the quantitative contribution of the various chaperone systems to overall protein folding and the mechanisms by which they select their substrates. As a long-term goal, we wish to understand chaperone usage at a proteome-wide level for the three branches of life.
Two major types of chaperones act in de novo folding in the cytosol: Nascent chain-binding chaperones, such as trigger factor, the Hsp70s and prefoldin, stabilize nascent chains on ribosomes in a folding-competent, non-aggregated state. Folding is either achieved upon controlled chain release from this first set of chaperones or upon chain transfer to chaperones that act downstream, such as the chaperonins. These are large, cylindrical complexes that provide a central compartment for a single protein chain to fold unimpaired by aggregation. These two classes of chaperone have been highly conserved in evolution and can cooperate in a topologically and timely ordered manner. However, the mechanism of this cooperation not yet well understood.
We are employing a range of methods from biophysics and structural biology to cell biology in order to understand the mechanisms of these chaperone systems. Translation and folding experiments are performed in bacterial and eukaryotic in vitro translation systems, as well as in Escherichia coli, budding yeast and mammalian cells in culture. Ultimately, we try to define a complex process in intact cells and then to reconstitute it in vitro at the level of purified components. Advanced proteomics methods are used to understand the function of the cellular chaperone machinery at a systems level.
Publications
Selected reviews
Hartl, F.U. and Hayer-Hartl, M. (2002). Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 295, 1852-1858.
Hartl, F.U.; Bracher, A., and Hayer-Hartl, M.: (2011). Molecular chaperones in protein folding and proteostasis. Nature 475, 324-332.
Kim, Y.E., Hipp, M., Bracher, A., Hayer-Hartl, M., and Hartl, F.U. (2013). Molecular chaperone functions in protein folding and proteostasis. Annual Rev. Biochem. 82, 323-355.
Balchin, D.; Hayer-Hartl, M., and Hartl, F.U. (2016). In vivo aspects of protein folding and quality control. Science 353, aac4354.
