Press Page F. Ulrich Hartl
It is a Matter of Form
Proteins acquire their correct conformation and can function properly only through folding. Misfolding can lead to diseases such as Alzheimer’s or Parkinson’s disease. But who ensures that nothing goes wrong? Franz-Ulrich Hartl and his team are interested both in the underlying mechanisms as well as the structure of the involved molecules.
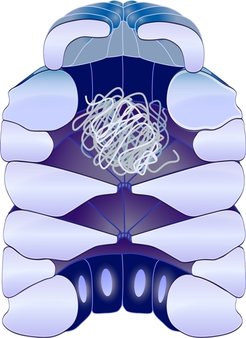
Proteins are molecules composed of long amino acid chains with a very complex three-dimensional structure. Hence, there are millions of possible ways to build these molecules – but only one way is correct and guarantees that the protein can fulfill its task. Proteins serving as “molecular chaperones” ensure that other proteins maintain their proper conformation. With their help, only the correct parts of the newly produced proteins come together to build a proper three-dimensional structure.
Chaperones at work
A special subgroup of the chaperones are the chaperonins. They form hollow cylinder-shaped complexes with a lid that look like a cage and provide a protected space for other proteins to fold. As shown for the chaperonin GroEL/GroES, these nano-cages not only shield proteins during folding, they also actively support the folding process. They may even repair misfolded proteins.
When folding goes awry
Misfolded proteins easily clump together to form insoluble aggregates which are then deposited in the cell and cause damage. Deposits of such protein aggregates are typical for neurodegenerative diseases such as Alzheimer’s, Parkinson’s or Huntington’s disease. Molecular chaperones can prevent this. Elucidating the structural requirements and mechanisms of chaperone activity may thus contribute to developing new drugs for the treatment of severe neurodegenerative diseases.



