Through the factory gate Sec61 into the membrane
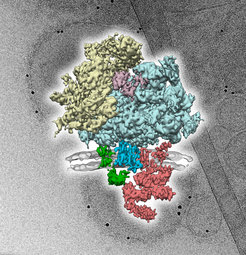
Proteins are assembled from amino acids in the ribosome (beige, light blue). They are then passed through the channel Sec61 (blue) and incorporated into the membrane (grey). Other components (green, red) are involved in the modification and folding of newly-synthesized proteins. The three-dimensional protein complex (foreground) was reconstructed from many two-dimensional images (background) that were taken with the cryogenic electron microscope.
Protein machines consist of long, twisted chains of amino acids. The assembly of these amino acids into proteins takes place in the ribosome. After assembly, around one third of all proteins get incorporated into cellular membranes where they operate, for example, as functional channels or receptors.
Not only the assembly of proteins is a big challenge, but also their incorporation into the membrane. Sec61 fulfils this task in all cells. Just like other proteins that are passed through the Sec61 channel, Sec61 is also incorporated into a membrane: a machine that helps constructing machines. Due to its central function, Sec61 is important for the general understanding of cellular function but also of diseases. For this reason, the analysis of the Sec61-structure had already been the target of numerous studies in the past. "In all previous studies, the channel had been removed from its natural membrane surroundings", reports Friedrich Förster, head of the research group 'Modeling of protein complexes'. “We wanted to find out whether this changed the structure and function of Sec61."
Förster's research group investigates Sec61 with the help of cryo-electron tomography. The researchers use a new, high-resolution detector system which enables them to analyze the structure of Sec61 in its natural membrane environment in unprecedented detail. The sample gets "shock frozen" to keep its natural shape. Afterwards, the researchers take two-dimensional images of the object from different viewing angles using the electron microscope. The images get subsequently reconstructed into a three-dimensional object. "This imaging technology functions in a similar way as the computer tomography in medicine", says Friedrich Förster. Using this methodology, natural structures can now be studied with a resolution of less than 1nm. This allows conclusions to be drawn almost down to the level of amino acids. "Astonishingly, Sec61 seems to behave differently within its natural membrane environment, than under isolation conditions. These results significantly change our understanding of how proteins get incorporated into the membrane", Förster summarizes the results of the current
Original publication:
Pfeffer, S., Burbaum, L., Unverdorben, P., Pech, M., Chen, Y., Zimmermann, R., Beckmann, R., Förster, F.: Structure of the native Sec61 protein-conducting channel. Nature Communincations, September 28, 2015
Doi: 10.1038/ncomms9403 (2015).


