Sweet proteins do better
Max Planck researchers uncover a new function of protein modifications
Many proteins in our cells are decorated with sugar molecule add-ons, which are essential for the functions of these proteins. One particular type of sugar modification, called GlcNAc, is of key importance, because our cells cannot survive without it. Researchers at the Max Planck Institute of Biochemistry in Martinsried near Munich have recently uncovered a previously unknown mechanism explaining how this sugar residue affects protein function and thereby influences our development. These results have been published in the journal Developmental Cell.
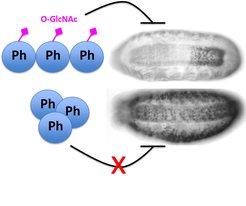
In wild-type Drosophila embryos, the Polyhomeotic (Ph) protein is O-GlcNAcylated (pink diamonds), and Ph maintains its target developmental patterning genes OFF in cells in which these genes are not supposed to be expressed. In animals lacking all O-GlcNAc on Ph, Ph forms large macromolecular aggregates that can no longer properly silence target genes.
Proteins are responsible for all vital processes in the cells of our body. However, they are not alone: tiny sugar molecules are decorating many proteins and they often are important to make them work properly. One particular type of sugar modification, called O-linked N-acetylglucosamine - in short called O-GlcNAc - seems to be of fundamental importance, because our cells cannot survive without it. Yet why exactly human cells die if their proteins lack the O-GlcNAc tag remains a mystery. Jürg Müller and Maria Cristina Gambetta at the Max Planck Institute of Biochemistry in Martinsried near Munich recently have addressed this question and set out to identify substrate proteins whose modification by O-GlcNAc is essential for biological processes. For their experiments the researchers made use of the less complex model organism Drosophila, also known as the fruit fly.
The researchers found that flies lacking O-GlcNAc show serious defects during their development: their cells fail to ‘remember’, which part of the body they were supposed to form. “Interestingly, we also observed the very same defects in flies lacking any member of the so-called Polycomb group of proteins” explains Maria Cristina Gambetta, the first author of the study. The Polycomb proteins permit cells to remember their fate by specifically silencing genes, which are not needed in those cells. However, how did these two results fit together? Why are the consequences of missing O-GlcNAc so similar to the consequences of missing Polycomb proteins?
The researchers were able to show that the function of one specific Polycomb protein, called Polyhomeotic - in short Ph - only functions properly if it carries the O-GlcNAc tag. The researchers found that the O-GlcNAc tag is critical to prevent Ph from forming large macromolecular clumps, which would interfere with its ability to silence its target genes. “This constitutes a previously unappreciated biochemical function of O-GlcNAc, namely to prevent the aggregation of a protein under normal physiological conditions”, explains Jürg Müller. “Moreover, we have been able to show that Polycomb repression is the most critical cellular process in flies that relies on O-GlcNAc.”
But how do these findings help us to advance our understanding of why our human cells need O-GlcNAc? In the latest study the researchers found out that fly and human Ph show high similarity. Consequently, also the human protein required O-GlcNAc in order to not clump together with other Ph proteins. “It will be interesting to further investigate, whether defective silencing of Polycomb target genes is also a major biological process that goes awry in human cells lacking O-GlcNAc”, Gambetta sets the agenda for the upcoming experiments. The results have recently been published in the journal Developmental Cell.
[HS]
Original Publication:
M.C. Gambetta and J. Müller: O-GlcNAcylation Prevents Aggregation of the Polycomb Group Repressor Polyhomeotic. Developmental Cell, November 26, 2014.
DOI:10.1016/j.devcel.2014.10.020






