Investigation of the function of halobacterial transducer proteins (Htrs) in the signal transduction machinery of Halobacterium salinarum
To sense its environment, the halophilic archeaeon Halobacterium salinarum employs a sophisticated protein machinery consisting of several types of components: External signals (light, chemicals, oxygen etc.) interact with receptor proteins which can be light receptors (e.g. sensory rhodopsins I and II), or binding proteins for chemicals (e.g. BasB or CosB) (Kokoeva, 2000 and Kokoeva, 2002). In other cases, the receptors can simultaneously be transducers (e.g. HemAT which is thought to bind oxygen with its heme group (Hou, 2000).
These receptor proteins forward the external signals via protein-protein interactions to halobacterial transducer proteins (Htrs). The Htrs - of which a total of 18 has been identified in H. salinarum by homology studies - transmit the external signal to an intracellular signaling pathway of Che proteins starting with the autophosphorylation of the histidine kinase CheA (Rudolph, 1995) which then phosphorylates the response regulator CheY. Phosphorylated CheY eventually induces a switch at the flagellar motor causing a reversal of flagellar rotational direction. This makes the cell reverse its swimming direction.
To enable the cells to adapt to a certain signal intensity and to respond only to changes in signal strength (e.g. changes in light intensity or substrate concentration), the Htrs are subject to methylation/demethylation of glutamate residues which are located in methylatable regions of the dimeric Htrs within a long helical coiled coil, whose structure was determined for Tsr, an Htr homolog in E. coli (Kim, 1999). Adaptation of the cells is achieved by changing the methylation state of the Htrs in response to external signals via the actions of the methyltransferase CheR and the methylesterase CheB, which is activated upon phosphorylation by CheA (reviewed in: Marwan, 1999).
In order to elucidate which role the Htrs are playing in environmental sensing, gene deletion approaches were chosen. Suicide-vectors with a mevinolin resistance marker were used to produce htr deletion mutants of H. salinarum. The deletion strains were validated by Southern blots and the strains were investigated by behavioural studies, e.g. by chemotaxis studies using soft agar plates (Storch, 1999 and Kokoeva, 2000).
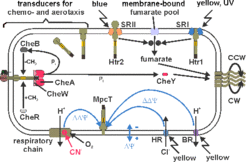
The picture above (taken from Koch, 2005) shows a schematic representation of our present view of Htr-mediated signaling in H. salinarum. In this organism, four retinal-containing photoactivatable proteins are present: the proton pump bacteriorhodopsin (BR), the chloride pump halorhodopsin (HR) and the sensory rhodopsins I and II (SRI and SRII). SRI and SRII confer phototaxis via HtrI and HtrII, respectively (reviewed in: Marwan, 1999). BR and HR are also involved in phototaxis (Bibikov, 1993, Bibikov, 1991, Grishanin, 1996). This happens via the generation of membrane potential changes (ΔΔΨ) across the membrane. These ΔΔΨ are maximal in the absence of functional respiration, as e.g. under oxygen depletion or in the presence of cyanide. MpcT (Htr14) was recently identified as the transducer responsible for ΔΔΨ sensing (Koch, 2005).
Other Htrs were shown to be involved in chemotaxis. The cytoplasmic protein Car (Storch, 1999) senses arginine, membrane spanning BasT (Kokoeva, 2000) confers chemotaxis towards the amino acids Met, Cys, Val, Leu and Ile via interaction with the binding protein BasB. CosT together with its binding protein CosB mediates chemotaxis towards compatible solutes like glycine betaine (Kokoeva, 2002). Htr8 and HemAT (Hou, 2000) were found to be involved in taxis towards oxygen.
The elucidation of the functions of the other Htrs in H. salinarum is one of the topics we are currently working on in our group.

The electronmicrograph above shows an H. salinarum cell with bipolar flagellation (taken from: Alam, 1984).

