retinal proteins of Halobacterium salinarum
I. Introduction
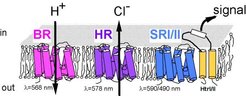
Halobacterium salinarum contains four different retinal proteins, the light-driven ion pumps BR (bacteriorhodopsin) and HR (halorhodopsin) as well as the phototactic receptors SRI (sensory rhodopsin I) and SRII (sensory rhodopsin II) (Fig.1)
Bacteriorhodopsin is an important model system for studying structure and function of a membrane protein which have been unraveled in great detail. It is one of the most intensively studied proteins due to application of a multitude of different experimental techniques.
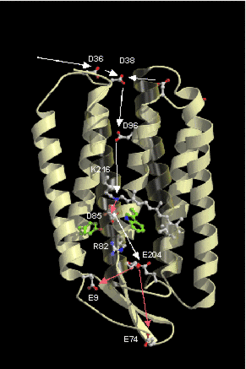
Retinal proteins are integral membrane proteins consisting of seven transmembrane helices containing retinal as prosthetic group (Fig.2 and Fig.3). Absorption of a single photon results in the photoisomerization of retinal from the all-trans to the 13-cis form. The 13-cis form is reverted to the all-trans form through several intermediates which can be identified by spectroscopic techniques.
The light-driven pumps BR and HR use the energy stored in the 13-cis form of retinal to drive ion transport across the membrane. The transition between photontermediates can be correlated with transfer of ions within the transmembrane pore.
The retinal proteins sensory rhodopsin I (SRI) and sensory rhodopsin II (SRII) are phototactic receptors. Here, the 13-cis form signals the activated state which is forwarded along the signal transduction cascade by specifically associated transducer proteins (htrI for SRI and htrII for SRII).
Important tools to study the structure and function of retinal proteins are spectroscopic techniques, X-ray structure analysis, and the possibility to produce specifically modified proteins by site directed mutagenesis. By this method the role of individual amino acid residues for the protein function can be investigated.


