The first structure of an archaeal ferritin from the halophilic microorganism Halobacterium salinarum
Corresponding publication:
K. Zeth, S. Offermann, L. -O. Essen, D. Oesterhelt:Iron-oxo clusters biomineralizing on protein surfaces: Structural analysis of Halobacterium salinarum DpsA in its low and high iron states. Proc. Natl. Acad.Sci. USA 101, 13780-13785 (2004)
Ferritins are one of the possibilities to overcome the problems with iron which have arisen since the increase of atmospheric oxygen: despite its abundance the cellular uptake of iron is inefficient, because under physiological pH and oxidizing conditions it occurs as Fe(III), which is largely insoluble. Furthermore, free Fe(II) ions generate highly reactive hydroxyl radicals by Fenton reactions with biogenic oxygen species such as superoxide anions or hydrogen peroxide (Harrison and Arioso, 1996). The ferritin proteins represent an iron storage in the cell which is capable to incorporate iron in a bioavailable and non-toxic form. They are characterized through a highly conserved architecture where generally 24 similar or identical subunits are assembled in a ball-like formation, each monomer exhibits a typical four-helix bundle fold. The iron is thought to be taken up as Fe(II), oxidized at the so called ferroxidase site within the helix bundle and accumulated as Fe(III) in a kind of crystalline conformation as ferrihydrite which has the approximate formula 5Fe2O3 x 9H2O (Chasteen and Harrison, 1999). Structural features of ferritins have been described from different eukaryotic and prokaryotic organisms but so far not from Archaea.
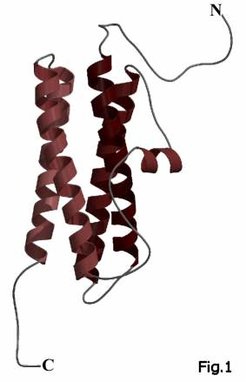
Here we crystallized the first archaeal ferritin from Halobacterium salinarum and solved the structure to a resolution of 1.8Å. The halobacterial ferritin shows the typical four-helix bundle as common structural feature of the ferritin monomers (Fig. 1), but contains 12 instead of 24 subunits (Fig. 2) like some recently discovered eubacterial ferritins for example from Listeria innocua (Ilari, 2001). Both proteins as well as some other 12-mer ferritins share an unusual intersubunit iron-binding site at the dimeric interface (Fig. 3) which seems to act as ferroxidase site. The ferritin from H. salinarum also illustrates how the adaptation to the hypersaline conditions of the haloarchaeal cytosol is achieved using a highly anionic protein surface, which constitutes a hallmark of halophilic proteins.


References
-
K. Zeth, S. Offermann, L. -O. Essen, D. Oesterhelt: Iron-oxo clusters biomineralizing on protein surfaces: Structural analysis of Halobacterium salinarum DpsA in its low and high iron states. Proc. Natl. Acad.Sci. USA 101, 13780-13785 (2004)
-
Harrison, P. M., and Arosio, P. (1996). The ferritins: molecular properties, iron storage function and cellular regulation. Biochimica et Biophysica Acta 1275, 161-203.
-
Chasteen, N. D., and Harrison, P. M. (1999). Mineralization in ferritin: an efficient means of iron storage. Journal of Structural Biology 126, 182-194.
-
Ilari, A., Stefanini, S., Chiancone, E., and Tsernoglou, D. (2000). The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site. Nature Structural Biology 7, 38-43


