Halobacterium salinarum uses the retinal protein bacteriorhodopsin, a light-driven proton pump, for photosynthesis. From this starting point, the interest of the department has extended to general aspects of bioenergetics. It was found that the organism shows an amazing flexibility with respect to its sources of energy.
Bioenergetics of Halobacterium salinarum
Halobacterium salinarum shows an amazing flexibility with respect to its energy sources: aerobic and anaerobic respiration, arginine fermentation, photosynthesis, and energy storage in ion gradients.
Retinal proteins of Halobacterium salinarum
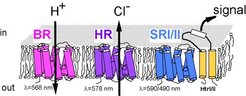
Halobacterium uses retinal proteins for photosynthesis and phototaxis.
Bacteriorhodopsin
Bacterirhodopsin, a retinal protein that functions as light-driven proton pump, allows photosynthesis of Halobacterium. As a consequence, the organism is able to grow with light as only source of energy.
Halorhodopsin
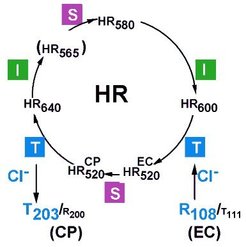
Halorhodopsin, a light-driven chloride pump, helps to establish the very high internal salt concentration, a characteristic of this halophilic organism. This allows the organism to use light energy rather than other energy sources that would be required for a classical ion pump.
Pyruvate--ferredoxin oxidoreductase and alpha-ketoglutarate--ferredoxin oxidoreductase
Enzymes ror oxidative decarboxylation of pyruvate and alpha-ketoglutarate use ferredoxin as coenzyme.
Ferredoxin from Halobacterium

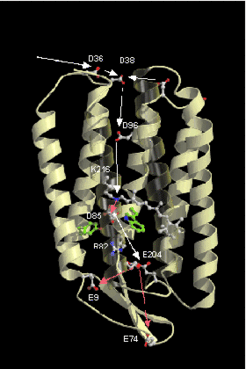
Ferredoxin is used as coenzyme for oxidative phosphorylation of pyruvate and alpha-ketoglutarate. Based on sequence comparison, the Halobacterium ferredoxin is closely related to ferredoxin from cyanobacteria. Its structure shows a unique acidic loop which may exemplify a rapid structural adaptation to cope with an extremely salty cytoplasm.




