1) Proteasome
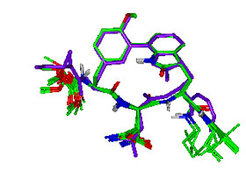
Members: M. Götz, V. Prasad (in collaboration with M. Groll, LMU München, and R. Huber, MPI)
Proteasomes are known to degrade ubiquitin-marked proteins. Additionally, proteasomes can be involved in peptide splicing, thereby generating non-contiguous antigenic peptides, which are finally presented by MHC class I molecules. TMC-95A, a specific reversible natural proteasome inhibitor, binds to the non-primed sites of the active centres in a substrate-like binding mode. The constrained conformation of TMC-95A provides the driving force for entropically high affinity binding. This structural framework was applied to the design and synthesis of biphenyl ether macrocycles, in which the biphenyl ether moiety functions as an endocyclic clamp that restricts the tripeptide backbone. Some biphenyl ether compounds were C-terminally extended with an amide group and were found to be prone to proteasome degradation. However, crystal structures of such proteasome-biphenyl ether complexes revealed proteolysis inhibition by an acyl-enzyme intermediate. Thus, substrates, leading to long-lived acyl-enzyme intermediates, can undergo an aminolysis mechanism induced by tightly binding proteolytic fragments. This unique mechanism explains the generation of an unexpected diversity of antigens. The formation of this intermediate was correlated with the strong plasticity of the tryptic-like binding cleft that augments for optimal fitting of inhibitors and substrates.
These results led to the design of a new generation of inhibitors with Cα-tetrasubstituted amino acids as P1 residues which should displace the nucleophilic water and thus form stable acyl-enzyme derivatives as a new type of largely irreversible proteasome inhibitors similar to the natural compounds lactacystin and salinosporamide A.
2) Cysteine-Proteases
Members: J. Pfizer, N. Schaschke (collaboration with W. Machleidt, C. Sommerhof, LMU München)
Financial support: DFG SCHA 1012/1
With epoxysuccinic acid as privileged structural element for irreversible inhibition of cysteine proteases libraries of the general structure R-NH-Leu-(2S,3S)-tEPS-Xaa-Yaa-OH were synthesized to screen representative members of the papain superfamily, particularly cathepsin B and L, for their subsite specificities. The results obtained in this study yielded interesting results for the further design of selective inhibitors of cysteine proteases. The concept of using the epoxysuccinyl moiety as reactive handle was further developed and applied for the design and synthesis of highly specific cathepsin L inhibitors as this group had already allowed the production of the most selective inhibitor of cathepsin B available so far. This inhibitor proved to be extremely useful for characterizing the physiological role of both intracellular and extracellular cathepsin B.
3) Tryptase
Members: N. Schaschke
Financial support: DFG SCHA 1012/1
Tryptase is still in the focus of synthesis and characterization of multivalent inhibitors using the natural product cyclotheonamide E with its known inhibitory properties against trypsin-like enzymes as a privileged structure. Both the synthesis of this cyclic peptide on resin and its correct dimerization is a challenging, but promising task for further progresses in bivalent inhibition of tryptases. Additionally, the construction of a tetravalent inhibitor on a suitable template capable of addressing simultaneously all four active sites of tryptase, should prove the limits accessible by the principle of multivalency.
4) Aspartyl-Proteases
Human β-secretase is still the main target in the laboratory among the aspartyl proteases. The design and synthesis of efficient inhibitors of aspartyl proteinases has been and still is a challenge, since mainly transition state analogues can be synthesized for this purpose. After more or less successful attempts to exploit peptoid structures for inhibiton of BACE more recently the main attention has been paid to the structure-based design of macrocyclic compounds containing a statin as transition state analogue and with the peptide backbone in an extended β-type conformation to reduce to a minimum the entropy penalty in the binding process. Although high affinity ligands could not be obtained so far, precious information was gained about the required fine tuning of a more or less rigid molecule to optimize its fitting into the active site cleft without perturbation of the protein surface. Complex macrocyclic molecules were synthesized with modulated flexibility which added further useful information for the iterative process of amelioration of binding affinities.
5) Metalloproteases
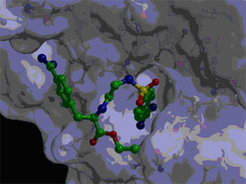
Members: A. Barazza, H.-J. Musiol (in collaboration with W. Bode, MPI)
The main approach in the design of MMP inhibitors was and still remains complexation of the active site metal ion. However, with this strategy it proved extremely dificult to produce highly selective inhibitors of the MMPs as required for their therapeutic use. As alternative a new approach was attempted with the generation of bivalent inhibitors that exploit both the active site binding and as exosite the binding site of fibrinogen on MMP-9 (gelatinase, an important target for rheumatic diseases). Complex structures were synthesized which are presently analyzed for their inhibition potency and are used in cocrystallization with MMP-9

