Members: S. Cadamuro, U. Kusebauch, M. Loeweneck, A. G. Milbradt, C. Renner, E. Weyher
Financial support: SFB 533
The promising results obtained in light-controlled conformational states of peptides backbone cyclized with (4-amino)phenylazobenzoic acid or (4-aminomethyl)phenylazobenzoic acid, our more recent interest was addressed to a possible photocontrol of tertiary structures. Such systems could yield precious information on the extremely fast processes of peptide and protein folding applying ultrafast UV and IR spectroscopy to monitor conformational transitions in the pico- to femtosecond time scale.
1) Light-triggered folding/unfolding of β-Hairpins

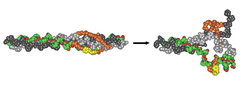
β-Hairpins constitute the smallest β-type structures and are often involved in stabilization of exposed loops for molecular recognition in proteins. We succeeded to design, synthesize and characterize a hairpin based on the tryptophan zipper motif which allowed for time-resolved folding studies of β-structures with unprecedented temporal resolution. At room temperature the trans-azo isomer exhibits a mostly disordered structure, but light-induced isomerization to the cis-azo form leads to a predominantly extended and parallel conformation of the two peptide parts, which are linked by the novel photo-switch [3-(3-aminomethyl)phenylazo]phenylacetic acid (AMPP). The β-hairpin structure was confirmed and determined by NMR spectroscopy, but folding can be monitored by pronounced changes in the CD, IR and fluorescence spectra. First ultrafast UV and IR spectroscopic data yielded the time scales of the successive steps in unfolding events which range from pico- to microseconds. These data will be used to attempt simulations of the folding/unfolding processes at least for such type of protein domain-structure. Incorporation of the structurally and functionally important β-hairpin motif into proteins by the methods of chemical ligation might lead to the photocontrol of protein structures and/or functions. For this purpose detailed analyses of the redox potential of azobenzene derivatives were performed to know the experimental conditions required in the native chemical ligation reactions as essential step for the assembly of such semisynthetic proteins
2) Light-triggered folding/unfolding of collagen triple helices
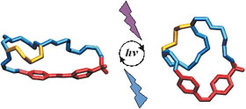
Since folding/unfolding of the collagen triple helix occurs in a two-state process, incorporation of a light-switch into this tertiary structure should lead to a facile monitoring of these processes in ultrafast time scales. For this purpose modeling experiments were performed to select the correct side chain positions in the (Gly-Pro-Hyp)N chains for grafting the azobenzene as a photoresponsive clamp capable of affecting the stability of the triple helix. Following the modelling experiments Cys residues were incorporated into selected positions of the collagen model peptides and a new azobenzene derivative was synthesized, i.e. azobenzene-4,4’-bis-N-(4-chloro-2-butynenyl)carboxamide which upon in situ conversion to the diiodo derivative, serves to crosslink intramolecularly the two cysteine side chains. Since already incorporation of two cysteines into (Pro-Hyp-Pro)7 was found to prevent triple helix formation (vide supra), in the ongoing project the model peptides containing larger N- and C-terminal extensions of (Pro-Hyp-Gly) triplets are synthesized to stabilize the triple helix.
Alternatively, the cysteine residues were replaced by mercaptoproline and crosslinked with the photoresponsive azobenzene derivative. By this process our dream was realized and for the first time a photocontrol of the collagen triple helix was realized which may well revolutionalize kinetics of polypeptide folding into ordered tertiary structures.
Unfolding of azobenzene containing collagen peptides: