1) Minicollagens
Members: C. Boulègue,A. G. Milbradt, C. Renner
Financial support: SFB 469, SFB 563
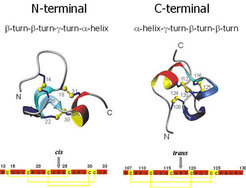
The main components of the capsule wall of nematocysts, the explosive organelles of Hydra, jellyfish, corals and other Cnidaria, are collagens which are termed minicollagens because of their shortest known collagen-like sequence of Gly-Xaa-Yaa repeats. Minicollagens are the major components of the Hydra nematocysts capsule wall where they form a tight three-dimensional network by disulfide reshuffling of their terminal cysteine-rich domains. Minicollagens contain a short collagen triple helix flanked by polyproline stretches and terminal cysteine-rich domains. These N- and C-terminal proline- and cystine-rich domains of minicollagen-1 are relatively short peptides (23 and 24 residues, respectively) containing 6 cysteine residues in an identical sequence pattern. The related synthetic peptides were found to refold oxidatively almost quantitatively into one topoisomer. But rather unexpectedly, the two refolded domains exhibit different cystine frameworks despite their identical cysteine pattern and a high sequence homology. Therefore the structural elements responsible for their distinct structures were analyzed to gain possible information about the mechanisms of minicollagen disulfide reshuffling in their assembly into polymeric fibers. With better information on this process, minicollagens containing functional domains of type I and IV collagens could be used for the preparation of mechanically highly resistant frameworks for cell adhesion and thus constitute promising innovative biomaterials.
NMR structures of cysteine rich domains:
Model of full minicollagen:

2) Self-assembly of collagenous peptides and the role of cysteines in native collagens
Members: S. Cadamuro, U. Kusebauch, H.-J. Musiol, C. Renner
Financial support: SFB 533, SFB 563
Cysteine residues are present in native collagens in non-triple helical portions where during maturation interchain disulfide knots are formed to crosslink the constituent three chains. This natural location of cysteine residues has been exploited in our previous studies for the assembly of C-terminally crosslinked homo- and heterotrimers of significantly enhanced thermostability using both artificial and natural cystine knots. However, single cysteine residues of unknown structural and/or biological function are also found in triple-helical sequence portions of collagens occupying both the Xaa and the Yaa positions of the Gly-Xaa-Yaa repeats. Host-guest studies performed in others and in our laboratory clearly revealed a strong triple-helix destabilizing effect which is more pronounced with cysteine residues in the Xaa than in the Yaa positions. Despite these destabilizing effects of cysteine residues, their role in native collagens could well be the crosslinking of the collagen fibers in the maturation process. To gain further structural information we have analyzed in the present study even the effect of a combined insertion of two cysteine residues in the Xaa and Yaa position. Moreover, to investigate whether collagenous sequence portions located between two cysteine residues form domains of lower structural stability, selectively 15N labelled collagenous peptides were synthesized and conformationally analyzed by both CD and NMR. In NMR the repetitive hydrogen bonding network between the Gly amides and the Pro carbonyls in Xaa positions of the adjacent chain which is critical for assembly and stability of the collagen triple helix, acts as an excellent index for this conformation, but also as a sensitive marker to assess the homotrimerization via NMR diffusion experiments.
Because of the observed destabilization of the triple helix by cysteine residues these were replaced by mercaptoproline which may retain to some extents the stereoelectronic effects of 4-hydroxyproline and thus allow formation of stable triple helices with the intrinsic advantage of containing thiol groups that can be exploited for crosslinking of collagen fibers and thus for increasing the mechanical strength of such artificial collagen.
Collagen triple helix:

3) Homotrimeric collagen peptides as cell adhesion epitopes
Members: S. Cadamuro, Hötzer, L., Renner, C., Sinner, E.-K.,
Based on previous studies with heterotrimeric collagen peptides as mimics of functional epitopes of collagen type I and IV, homotrimers crosslinked C-terminally by the natural cystine knot of collagen type III and containing the cell adhesion epitope of collagen type I were synthesized, conformationally characterized and assayed for their ability to bind the recombinanty produced a1 I domain of α1β1-Integrin. The positive results obtained with the triple-helical homotrimer are compelling for attempting the next step in the design of soft surfaces by built-in lipophilic moieties that allow for directly sequestering collagen peptides on lipid bilayers constructed on soft surfaces. This could then be used to study and monitor intact cell adhesion processes with suitable spectroscopic devices.
Formation of the natural cystine knot: