New molecule in gear box of cells
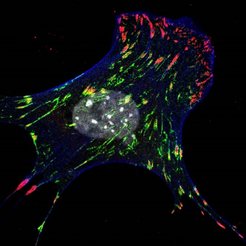
The picture shows a cell, in which the newly discovered protein Kank2 is shown in green. Integrin-based adhesions (in red) strongly anchor to the extracellular matrix at the cell edges but loose the grip in the cell center where Kank2 is present. Contractile actin cytoskeleton is shown in blue. In the center the cell nucleus is visible.
In tissue, cells are embedded within the extracellular matrix, a meshwork of different protein assemblies. Integrins are anchor proteins on the cell surface, which on the one hand enable cells to adhere to the extracellular matrix, and on the other hand connect to the actin cytoskeleton inside the cells. Hence, integrin-mediated adhesion points transmit mechanical force generated by the contractile cytoskeleton to the extracellular matrix, which is needed for cell migration. The stronger integrin-based adhesions are pulled by mechanical force, the tighter they are anchored on the extracellular matrix.
Similar to the gearbox of a driving car, many integrin- and actin-binding proteins including Talin are needed to clutch the actin engine with integrins. Scientist of the department “Molecular Medicine” at the Max Planck Institute for Biochemistry discovered the Kank protein family as a new component within the cellular gearbox. “At integrin-based adhesions, Kank2 reduces the connection between Talin and the actin cytoskeleton and hence diminishes the force transmission across integrins”, explains Zhiqi Sun, first author of the study. Consequently, the grip between the cell and the matrix is reduced– similar to a car on an icy street. “We have discovered a kind of gearshift. Depending on the Kank protein, the anchorage strength and eventually the speed of the cell movement can be changed”, explains Sun additionally.
The newly discovered mechanism may be especially interesting for the treatment of cancer. “If one could actively influence the clutch engagement in the gearbox of tumor cells, we would have a new tool to probably slow disease progression”, summarizes Zhiqi Sun.
Original Publication:
Z. Sun, H.-Y. Tseng, S. Tan, F. Senger, L. Kurzawa, D. Dedden, N. Mizuno, A. A.Wasik, M. Thery, A. R. Dunn and R. Fässler: Kank2 activates talin, reduces force transduction across integrins and induces central adhesion formation, Nature Cell Biology, August 2016
DOI: 10.1038/ncb3402

